Press Releases
Annual Cost of False-Positive Breast Biopsies Exceeds $2 Billion According to Recent Study
May 30, 2018– Publication in ClinicoEconomics and Outcomes Research estimates nearly $8 billion annually in expense for additional breast diagnostic testing, underscoring market need for improved breast diagnostic tools –
SAN ANTONIO, May 31, 2018 – False-positive breast biopsies in the United States cost the healthcare system more than $2 billion per year, according to findings released from a new study in ClinicoEconomics and Outcomes Research, conducted by a team of health outcomes research scientists at IBM Watson Health™ in collaboration with Seno Medical’s Chief Medical Officer, A. Thomas Stavros, MD.1
The study analyzed recent data focusing on diagnostic breast imaging, biopsies, and other diagnostic procedures performed for patients recalled for follow-up after suspicious findings from initial screening mammography or breast examination. Such follow-up procedures are ordered by clinicians largely because current tools often cannot provide diagnostic certainty in identifying cancerous breast masses. The study looked at how often the follow-up procedures were performed, on what volume of patients, the most common sequences in which the procedures were performed, and the associated costs.
Data was collected from a nationally representative sample of 875,000 adult women using real-world encounters from health care claims data from 2011 to 2015, and then projected nationally to estimate that more than 12 million women in the US received follow-up exams each year after suspicious findings. Based on actual payer claims for the women studied, 53.3% of such patients received diagnostic mammograms, 42.4% received diagnostic breast ultrasounds, and 10.3% received biopsies after initial diagnostic procedures. Total costs for these procedures were projected at:
- $3.05 billion for diagnostic mammograms (average cost $349)
- $0.92 billion for diagnostic breast ultrasounds (average cost $132)
- $3.07 billion for biopsies (average cost $1,938)
Combined with other follow-up imaging procedures (e.g., tomosynthesis, MRI, others), the data totals nearly $8 billion spent annually for follow-up breast diagnostic procedures. [See Infographic.]
Breast cancer is the most common malignancy among women worldwide and the second leading cause of cancer-related deaths in females, and it is estimated that 266,120 new cases of invasive breast cancer will be diagnosed in women in 2018.2 Survival rates have increased steadily over recent decades as earlier detection enables treatment at earlier stages when treatment is more effective and less costly. However, many organizations differ on timing (annually, bi-annually) and ages for screening (to begin at age 40 or 50 years, screening after age 74 years), with varying opinions on how to best balance breast cancer screening costs with rates of detection, rates of false-positives or over diagnoses, and reduction in mortality.1
The study authors stressed the critical need for follow-up with patients who present with abnormal results on a screening mammogram. Standards of care and practice guidelines require further imaging studies before an invasive procedure such as breast biopsy, when the screening mammogram uncovers something suspicious.
They further recognized unmet medical need for highly effective exam tools that could exclude patients whose suspicious breast masses are benign before they are subjected to invasive diagnostic procedures. Breast biopsies have been found to show a false-positive rate following diagnostic screening procedures as high as 71 percent in the United States according to the National Cancer Institute3, translating to an annual cost of $2.18 billion in biopsy procedures that might have been avoided.
“The costs to the healthcare system are secondary to the psychological impact on women who are told that their mammogram and ultrasound were inconclusive, and that a biopsy is required to rule out cancer,” says A. Thomas Stavros, MD, FACR, FSRU, FRANZCR, Professor Specialist, Department of Radiology University of Texas Health Sciences Center and Chief Medical Officer of Seno Medical, San Antonio, TX.
Stavros continued, “Conscientious clinicians rightly want to confirm that a mass is not malignant, so the guidelines and clinical practice aren’t at fault. It’s simply that technology – as advanced as it has become – still needs further refinement to provide better specificity without sacrificing sensitivity and to engender increased diagnostic confidence for the clinician. There are significant volumes and costs of procedures required to reach a definitive, “yes,” that breast cancer does or does not exist.”
“Our findings bring a national spotlight on the current diagnostic procedure journey faced by patients and providers in order to have confidence in the evidence of a woman’s risk of breast cancer,” says Jay Margolis, PharmD, Senior Research Scientist, Life Sciences, Value Based Care at IBM Watson Health and senior author for the study. “Recalling a woman for subsequent imaging procedures that may not be truly needed places significant burdens on the patient, her family, and her career, with substantial costs to the healthcare system.”
About Seno Medical Instruments, Inc.
Seno Medical Instruments, Inc. is a San Antonio, Texas-based medical imaging company committed to the development and commercialization of a new modality in cancer diagnosis: opto-acoustic imaging. Seno Medical’s Imagio™ breast imaging system fuses opto-acoustic technology with ultrasound (OA/US) to generate fused real-time functional and anatomical images of the breast. The opto-acoustic images provide a unique blood map around breast masses while the ultrasound provides a traditional anatomic image. Through the appearance or absence of two hallmark indicators of cancer – angiogenesis and deoxygenation – Seno Medical believes that the Imagio OA/US breast imaging system will be a more effective tool to help radiologists confirm or rule out malignancy than current diagnostic imaging modalities – without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. To learn more about Seno Medical’s OA/US imaging technology, visit www.SenoMedical.com.
1 Vlahiotis A, Griffin B, Stavros AT, Margolis J. Analysis of Utilization Patterns and Associated Costs of the Breast Imaging and Diagnostic Procedures After Screening Mammography. ClinicoEconomics and Outcomes Research 2018:10 157-167.
2 American Cancer Society, Current year estimates for breast cancer, January 4, 2018 http://ibm.biz/BdZJvc
3 NCI-funded Breast Cancer Surveillance Consortium (HHSN261201100031C). Downloaded from the Breast Cancer Surveillance Consortium Web site – http://breastscreening.cancer.gov/.http://www.bcsc-research.org. Accessed January 23, 2017.
Media Contact
Erich Sandoval
Lazar Partners Ltd.
Tel: +1 917-497-2867
Email: esandoval@lazarpartners.com
New Analytics Approach Offers Potential of Reducing Unnecessary Breast Biopsies
May 02, 2018– Publication in AJR defines advanced statistical methods that can be used with diagnostic imaging output to downgrade breast mass risk classification –
SAN ANTONIO, May 3, 2018 – The American Journal of Roentgenology recently published findings on statistical methods for downgrading the risk classification of breast masses to reduce the need for unnecessary breast biopsies. Clinicians from Seno Medical and medical center collaborators from The University of Texas co-authored the report.1
“The perceived risk of missing a breast cancer diagnosis with breast imaging studies is much higher than the risk of a false-positive diagnosis, leading to breast imagers recommending a breast biopsy whenever the risk of cancer is greater than 2%. Sometimes ancillary diagnostic breast imaging studies are performed to reduce risk to less than 2%, but it is difficult to know exactly how much risks are reduced even after a negative ancillary diagnostic imaging examination,” said Thomas Stavros, MD, Chief Medical Officer of Seno Medical and a co-author of the report. “However, the use of the Negative Likelihood Ratio (NLR) along with BI-RADS 4 subcategories can help to reduce the number of false-positives without experiencing excessive negative results that would lead to cancer going undiagnosed.”
The report explores the use of a statistical calculation known as the Negative Likelihood Ratio (NLR). It shows how NLR can be calculated from a diagnostic test’s sensitivity and specificity and also show the NLRs of some currently available diagnostic imaging modalities. It outlines how the BI-RADS (Breast Imaging and Reporting Data System, or BR) 4A sub-category has low enough and narrow enough range of pre-test probabilities (see Table 1) to allow downgrading to a post-test probability of 2% or less after a negative diagnostic imaging test with an adequately low NLR.
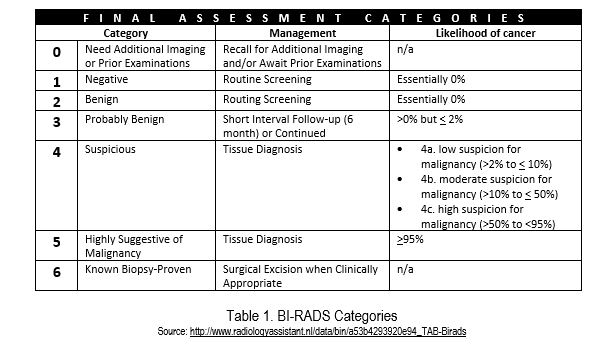
Each BI-RAD category is associated with a specific risk of breast cancer. Briefly, the approach includes the following steps:
- Classify lesions according to BI-RADS category 4 subcategories. Subcategory 4A is important as it represents the subcategory where the range of PPVs is both low enough and narrow enough to allow downgrading to BI-RADS category 3 is most possible when a diagnostic imaging test result is negative.
- Confirm that the Positive Predictive Value (PPV) is within the American College of Radiology (ACR) benchmark PPV range for BI-RADS subcategory 4A (>2% to 10%).
- Ensure the NLR is adequate for a negative test finding to reduce the post-test probability to 2% or less.
“Reducing the number of unnecessary breast biopsies is an essential advancement toward improving women’s healthcare and protecting breast health,” said Pam Otto, M.D., Department of Radiology, The University of Texas Health Science Center at San Antonio and co-author. “I would encourage breast imagers to consider using BI-RADS 4 subcategories and NLR as important tools for helping them minimize false positive studies with minimum adverse effect on sensitivity, optimizing their patients’ breast health. The availability of web-based programs for automating the NLR calculations should help to facilitate routine use of this important statistical tool.”
About Seno Medical Instruments, Inc.
Seno Medical Instruments, Inc. is a San Antonio, Texas-based medical imaging company committed to the development and commercialization of a new modality in cancer diagnosis: opto-acoustic imaging. Seno Medical’s Imagio™ breast imaging system fuses opto-acoustic technology with ultrasound (OA/US) to generate fused real-time functional and anatomical images of the breast. The opto-acoustic images provide a unique blood map around breast masses while the ultrasound provides a traditional anatomic image. Through the appearance or absence of two hallmark indicators of cancer – angiogenesis and deoxygenation – Seno Medical believes that the Imagio OA/US breast imaging system will be a more effective tool to help radiologists confirm or rule out malignancy than current diagnostic imaging modalities – without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. To learn more about Seno Medical’s OA/US imaging technology and applications, visit www.SenoMedical.com.
[1] Yang WT, Parikh JR, Stavros AT, Otto P and Maislin G. Exploring the Negative Likelihood Ratio and How It Can Be Used to Minimize False-Positives in Breast Imaging. AJR 2018;210:301-306.
Opto-Acoustic Imaging Leader Seno Medical Instruments Announces Recipient of Its 8th Annual Best Paper Award during the SPIE Annual Conference
January 29, 2018-2017 award recognizes a novel optical imaging modality that provides high molecular contrast of
important biomarkers throughout the body –
SAN ANTONIO, January 30, 2018 – Seno Medical Instruments, Inc. (Seno Medical), the leader in new technology for breast cancer diagnosis using opto-acoustic (OA/US) imaging to differentiate benign from malignant masses, today announced the winner of the 2017 Best Paper Award at the International Society for Optics and Photonics (SPIE) “Photons Plus Ultrasound: Imaging and Sensing” annual conference. This year’s award was given to a team of researchers from California Institute of Technology, Washington University in St. Louis, Duke University and Tsinghua University, Beijing who have developed a novel photoacoustic technology. Seno Medical has sponsored the award for the past eight years, which includes a Certificate of Accomplishment and a $3,000 cash prize.
The conference, held under the auspices of the SPIE/BIOS Photonics West symposium, is the largest international forum for the biomedical opto-acoustics research community, held this week in San Francisco January 27th – 31st. More than 250 papers representing the latest results in imaging and sensing technologies, including both clinical and pre-clinical applications, are presented.
The field of biomedical opto-acoustic (photoacoustic) imaging continues to experience rapid growth. In 2017, the total number of research papers published in peer-reviewed literature on the subject of opto-acoustic imaging exceeded 500, and the total number of papers published on the technology now exceeds 5,000. The number and sophistication of papers reporting on opto-acoustic clinical applications and commercial grade systems have been increasing since the beginning of the 21st century, a trend that became even more prominent at this year’s conference. There are approximately 1,000 attendees at the symposium, and the clinical application sessions achieved standing-room-only attendance.
“As a leader in applying opto-acoustic imaging to advance the diagnosis of breast cancer, we know firsthand that these technologies have tremendous potential in improving patient care and outcomes,” said Steven Miller, Senior Vice President of Engineering at Seno Medical. “Seno Medical is committed to fostering innovation that helps advance patient care, and we are excited about the increased number and sophistication of papers reporting on opto-acoustic clinical applications and commercial grade systems at this year’s conference. The technology described in the award-winning paper has the potential to significantly improve the resolution of whole-body dynamic imaging for small animals used in many areas of medical research from pathology to oncology.”
The 2017 Best Paper Award was given to the team for their work on the study Imaging Small-animal Wholebody Dynamics by Single-impulse Panoramic Photoacoustic Computed Tomography. Seno Medical congratulates the papers’ authors, Lei Li, Liren Zhu, Cheng Ma, Li Lin, Junjie Yao, Lidai Wang, Konstantin Maslov, Ruiying Zhang, Wanyi Chen, Junhui Shi, and Lihong V. Wang. The company also thanks the Organizing Committee and the contributors to this conference for their ongoing efforts to support the transformation of biomedical opto-acoustics and photoacoustics into clinical applications that can address real unmet needs within the healthcare system.
The Single-impulse Panoramic Photoacoustic Computed Tomography (SIP-PACT) technology substantially enhances imaging performance to complement existing modalities for small animal whole-body imaging. This technology non-invasively images mouse anatomy real-time, with clearly viewed sub-organ vasculature and structure. SIP-PACT as a whole-body imaging tool for small animals will enable widespread applications in fundamental biology, pathology, oncology and other areas.
Two other papers were selected as finalists for the 2017 award:
- Photoacoustic analysis of thyroid cancer in vivo: A pilot study, by Jeesu Kima, Min-Hee Kimb, Kwanhoon Jo, Jeonghoon Hab, Yongmin Kim, Dong-Jun Lim, and Chulhong Kim
- Possibility of transrectal photoacoustic imaging-guided biopsy for detection of prostate cancer, by Miya Ishihara, Masayuki Shinchib, Akio Horiguchi, Hiroshi Shinmotoc, Hitoshi Tsudad, Kaku Irisawae, Takatsugu Wadae, and Tomohiko Asanob.
About Seno Medical Instruments, Inc.
Seno Medical Instruments, Inc. is a San Antonio, Texas-based medical imaging company committed to the development and commercialization of a new modality in cancer diagnosis: opto-acoustic imaging. Seno’s Imagio™ breast imaging system fuses opto-acoustic technology with ultrasound to generate functional and anatomical images of the breast. The opto-acoustic images provide a unique color map in and around suspicious breast masses while the ultrasound provides a traditional anatomic image. Through the appearance or absence of the two hallmark indicators of cancer – angiogenesis and deoxygenation – Seno believes that Imagio™ images will be a more effective tool to help radiologists confirm or rule out malignancy than current diagnostic imaging modalities – without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. Seno’s platform technology may also address other disease applications in organs other than the breast, as well as assessing other breast problems, such as early response to neoadjuvant chemotherapy or hormonal treatments of breast cancer. To learn more about Seno’s opto-acoustic imaging technology and applications, visit www.SenoMedical.com
Seno Medical Instruments Announces New Data Demonstrating That Imagio™ Opto-Acoustic Breast Imaging System Delivers Critical Diagnostic and Prognostic Information About Tumor Subtypes
December 07, 2017– Prospective clinical study presented at the San Antonio Breast Cancer Symposium suggests Imagio findings may help clinicians tailor patient treatment regimens –
– Functional Imaging provides important information about the growth and location of blood vessels around breast masses as compared to anatomical imaging-
SAN ANTONIO, December 8, 2017 – Seno Medical Instruments, Inc. (Seno Medical), the leader in new technology for breast cancer diagnosis using opto-acoustic (OA/US) imaging to differentiate benign from malignant masses, reported clinical data from their clinical study demonstrating that its Imagio™ breast imaging system achieved significant differentiation among prognostic subtypes of breast cancer (PSBC). These prognostic subtypes provide important information about the genes that each patient’s cancer expresses. The data are being presented today at 5:00 p.m. during a poster presentation [#P5-02-04] at the San Antonio Breast Cancer Symposium taking place December 5 – 9.
“Confirming a diagnosis of breast cancer is just the first step in understanding the pathology of a patient’s tumor,” said study presenter Stephen Grobmyer, M.D., Director, Breast Surgical Oncology, Cleveland Clinic. “Determining a patient’s prognostic subtype of breast cancer is essential for understanding how tumors will respond to specific therapies and selecting a treatment regimen that offers the patient the best chance of a positive outcome. The findings that OA/US may differentiate among several breast cancer subtypes suggest that this novel imaging methodology could potentially provide a non-invasive alternative to biopsy without sacrificing information about the molecular pathology of the tumor. This could be an important advance for women with breast cancer.”
The prospective, multi-center study was conducted at 16 centers in the United States. A total of 1,808 breast masses in 1,739 subjects assessed as BI-RADS (Breast Imaging and Reporting Data System, or BR) category 3, 4 or 5 were imaged with OA/US, resulting in the identification of 678 malignant lesions. Eight blinded readers scored these 678 lesions based on three internal features of the tumor and two external features of the tumor boundary and surrounding tissue. An experienced central breast pathologist who was blinded to the OA/US assessment confirmed the pathologic diagnoses. Biopsy specimens were obtained and assessed for the expression of four genes that comprise the PSBC: estrogen receptor (ER), progesterone receptor (PR), HER2-neu (HER2) and Ki-67. The PSBCs are defined as follows:
- Luminal A (LUM-A): ER/PR (+) and Ki-67 and HER2 (-). These tumors are often low grade, with slow tumor growth and are associated with the most positive prognosis.
- Luminal B (LUM-B): ER (+), PR (+/-), Ki-67 (+) and HER2 (+/-). These tumors are also low grade but grow more quickly than LUM-A.
- Triple-negative breast cancer (TNBC): ER/PR (-), Ki-67 (+/-) and HER2 (-). These tumors are aggressive and typically associated with poor prognosis.
- HER2-enriched (HER2): ER/PR (-), Ki-67 (+/-) and HER2 (+). These tumors tend to grow quickly but are responsive to anti-HER2 targeted therapies.
Tumors not meeting the definition of any of these categories were deemed unclassified.
Key findings from the study include:
- There was a significant difference in the sum of the three internal OA/US features between LUM-A and TNBC subtypes (p = 0.0256) and borderline significance between LUM-A and LUM-B (p = 0.0831) and LUM-A and HER2 (p = 0.0707).
- There was a significant difference in the sum of the two external OA/US features between LUM-A and TNBC subtypes (p = 0.0001) and LUM-B and TNBC subtypes (p = 0.0020) and borderline significance between LUM-A and HER2 (p = 0.0617).
- TNBC tumors showed mainly internal findings consisting of polymorphic intensely deoxygenated vessels.
- LUM-A tumors showed a paucity of internal findings and abundant external findings consisting of short perpendicularly oriented tumor vessels in the boundary zone (BZ whiskers), partial BZ deoxygenated blush, and peripheral radiating deoxygenated vessels.
“The results of this study underscore the clinical benefits that can be achieved with Imagio’s functional imaging technology,” said Tom Umbel, CEO of Seno Medical Instruments. “Unlike anatomical imaging techniques, such as mammography or conventional ultrasound, functional imaging provides important information about the growth and location of blood vessels in and around breast masses. These findings show that not only does this functional imaging data allow for more accurate diagnosis of breast cancer, it also correlates with existing prognostic breast cancer subtypes. This data may suggest that OA/US has the potential to provide physicians with a powerful tool for gaining insight into the molecular pathology of each patient’s tumor without the need for invasive biopsy procedures. Seno Medical is committed to improving the care and outcomes of women with breast cancer through improved non-invasive diagnostic approaches.”
The Imagio™ OA/US breast imaging system was designed and is being studied to identify two functional hallmarks of cancer: the presence of abnormal blood vessels (tumor angiogenesis) and the relative reduction in oxygen content of blood that occurs in cancer compared to benign masses and normal tissues. The technology is the subject of a U.S. PMA filing with the FDA and does have European CE Mark.
About Seno Medical Instruments, Inc.
Seno Medical Instruments, Inc. is a San Antonio, Texas-based medical imaging company committed to the development and commercialization of a new modality in cancer diagnosis: opto-acoustic imaging. Seno Medical’s Imagio™ breast imaging system fuses opto-acoustic technology with ultrasound (OA/US) to generate fused real-time functional and anatomical images of the breast. The opto-acoustic images provide a unique blood map around breast masses while the ultrasound provides a traditional anatomic image. Through the appearance or absence of two hallmark indicators of cancer – angiogenesis and deoxygenation – Seno Medical believes that the OA/US breast imaging system will be a more effective tool to help radiologists confirm or rule out malignancy than current diagnostic imaging modalities – without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. To learn more about Seno Medical’s OA/US imaging technology and applications, visit www.SenoMedical.com.
About Breast Cancer
According to the American Cancer Society, an estimated 246,660 new cases of invasive breast cancer, along with 61,000 new cases of non-invasive (in situ) breast cancer, will be diagnosed in U.S. women in 2016. An estimated 40,450 women in the U.S. are expected to die in 2016 from breast cancer. Only lung cancer accounts for more cancer deaths in women.[1]
[1] U.S. Breast Cancer Statistics | Breastcancer.org. (2016). Breastcancer.org. http://www.breastcancer.org/symptoms/understand_bc/statistics
Seno Medical Instruments’ Imagio™ Opto-Acoustic Breast Imaging System Demonstrates Ability to Downgrade Risk Classification of Benign Breast Masses in a Prospective European Clinical Trial
November 27, 2017CHICAGO, November 28, 2017 – Seno Medical Instruments, Inc. (Seno Medical), the leader in new technology for breast cancer diagnosis using optoacoustic (OA/US) imaging to differentiate benign from malignant masses, today announced clinical data demonstrating that its Imagio™ breast imaging system effectively downgraded the risk classification of benign breast masses and upgraded the risk classification of malignant masses compared with ultrasound (US) alone. The data are being presented today at 3:20 p.m. CDT during an oral presentation by a principal study investigator at the Radiology Society of North America 103rd Annual Meeting, taking place November 26 – December 1 in Chicago.
The prospective, controlled, multi-center study was conducted at five centers in the Netherlands between March 2015 and February 2016 and enrolled 217 patients at least 18 years of age with at least one breast mass totaling 223 breast masses classified as BI-RADS (Breast Imaging and Reporting Data System) category 4A or 4B and with a corresponding probability of malignancy (POM) ranging from >2% to 50% based on prior US assessment. The likelihood that a breast mass is malignant increases with increasing BI-RADS category number. All subjects underwent an OA/US evaluation after conventional US and prior to biopsy. The OA/US data were used to estimate the POM on a scale from 0% to 100% and, when appropriate, the BI-RADS classification determined by conventional US was adjusted. Biopsies were performed within 30 days of enrollment and after the OA/US procedure had been performed and interpreted. The OA/US results were compared with biopsy results as the reference standard. Patients were asked to complete a satisfaction survey after undergoing OA/US evaluation
“The negative consequences of biopsies of benign breast masses include pain, anxiety, fear, direct expenses and indirect expenses related to work missed and managing complications,” said study investigator Gisela L.G. Menezes, M.D., Ph.D. and postdoc at the University Medical Center Utrecht, the Netherlands. “Using OA/US, benign masses originally classified as suspicious may be downgraded to probably benign or benign, potentially decreasing the number of biopsies of benign lesions.”
Key findings from the study include:
- In the intent to diagnose (ITD) population of 209 patients with 215 masses, 68% were benign, 31.1% were malignant and 0.9% were considered high-risk lesions based on histopathology of biopsy specimens.
- Based on US of 146 benign lesions, 81.5% and 18.5% were classified as BI-RADS 4A and 4B, respectively. Sixty benign masses were correctly downgraded from BI-RADS 4A or 4B to BI-RADS 3 or 2 with OA/US (p<0.0001).
- Of benign masses classified as BI-RADS 4A, 47.9% were downgraded to BI-RADS 3 or 2 and 12.3% were upgraded to 4B.
- Of 27 benign masses classified as 4B, 11.1% were downgraded to BI-RADS 3, 6.1% were upgraded to 4C and 0.6% were upgraded to 5.
- Overall, 41.1% of benign breast masses originally classified as BI-RADS 4A or 4B on US could be downgraded to probably benign or benign masses (BI-RADS 3 or 2) with OA/US, and 49.2% of malignant breast masses could be upgraded to a higher BI-RADS category.
- The OA/US true positive rate was 95.5%.
- There were no safety issues related to OA/US.
- The patient satisfaction survey showed that approximately 95% of the patients agreed that OA/US had an acceptable level of comfort and 84% agreed that OA/US scan time was acceptable.
“The results from this study indicate that a substantial percentage of breast masses classified as BI-RADS 4A or greater, based on conventional US, can be downgraded with OA/US. This suggests that the functional and morphologic information gained with OA/US has the potential to benefit women undergoing breast mass evaluation,” said Tom Umbel, CEO of Seno Medical Instruments. “Detection of a breast mass can make a woman very anxious, and it is incumbent on everyone involved in assessing breast masses to minimize patients’ stress and anxiety while providing the most accurate diagnosis possible. We believe that the Imagio system has an important role to play in improving breast cancer diagnosis and ensuring optimal patient care and outcomes.”
The Imagio™ breast imaging system was designed and is being studied to identify two functional hallmarks of cancer: the presence of abnormal blood vessels (tumor angiogenesis) and the relative reduction in oxygen content of blood that occurs in cancer compared to benign masses and normal tissues. The technology has its CE mark in Europe and is the subject of a U.S. PMA filing with the FDA.
About Seno Medical Instruments, Inc.
Seno Medical Instruments, Inc. is a San Antonio, Texas-based medical imaging company committed to the development and commercialization of a new modality in cancer diagnosis: opto-acoustic imaging. Seno Medical’s Imagio™ breast imaging system fuses opto-acoustic technology with ultrasound (OA/US) to generate fused real-time functional and anatomical images of the breast. The OA/US images provide a unique blood map around breast masses while the ultrasound provides a traditional anatomic image. Through the appearance or absence of two hallmark indicators of cancer – angiogenesis and deoxygenation – Seno Medical believes that the OA/US breast imaging system will be a more effective tool to help radiologists confirm or rule out malignancy than current diagnostic imaging modalities – without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. To learn more about Seno Medical’s OA/US imaging technology and applications, visit www.SenoMedical.com.
About Breast Cancer
According to the American Cancer Society, an estimated 252,710 new cases of invasive breast cancer, along with 63,410 new cases of non-invasive (in situ) breast cancer, will be diagnosed in U.S. women in 2017. An estimated 40,610 women in the U.S. are expected to die in 2017 from breast cancer. Only lung cancer accounts for more cancer deaths in women.[1]
[1] Breast Cancer Facts & Figures, American Cancer Society (2017). Accessed on October 24, 2017: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html
Pivotal Trial Data Support Potential of Seno Medical Instruments’ Imagio™ Opto-Acoustic Breast Imaging System in Reducing False Positive Breast Examinations and Benign Biopsies
November 26, 2017Results Presented in a High Impact Clinical Trial Session at the Radiology Society of North America (RSNA) Annual Meeting
CHICAGO, November 27, 2017 – Seno Medical Instruments, Inc. (Seno Medical), the leader in new technology for breast cancer diagnosis using opto-acoustic ultrasound (OA/US) imaging to differentiate benign from malignant masses, today announced positive data from PIONEER, a Phase III pivotal trial of its Imagio™ breast imaging system. The study found that OA/US was more specific than device gray-scale ultrasound alone (US) in differentiating malignant from benign breast lesions and was non-inferior to US with respect to sensitivity. This means that OA/US detected a similar number of malignant lesions as US but had a lower rate of false positive malignant diagnoses. These data were highlighted during an oral presentation by a principal investigator involved with the study at the Radiology Society of North America 103rd Annual Meeting, taking place November 26 – December 1 in Chicago.
PIONEER was a U.S., prospective, multi-institutional study that enrolled 2,105 women over the age of 18 years. All participants had a breast mass assessed by initial conventional site ultrasound as BI-RADS (Breast Imaging and Reporting Data System, or BR) category 3, 4, or 5. The likelihood that a breast mass is malignant increases with increasing BR category number. Radiologists at each clinical site also assigned each mass a probability of malignancy (POM) ranging from 3% to 99% based on mammographic and US features. Patients with masses categorized as > BR4A had OA/US prior to undergoing core needle biopsy within 45 days of their initial visit and subsequent biopsy or excision surgery within 45 of the initial biopsy. Patients with BR3 masses had OA/US upon enrollment and underwent biopsy or conventional site US and OA/US 12 months after enrollment. Patients who underwent biopsy due to new clinical or imaging findings within the 12-month period had additional OA/US imaging within 45 days prior to the biopsy. Independent breast imagers who were blinded to the biopsy and other clinical information read the OA/US and device US images; an independent central pathologist who was blinded to the imaging data reviewed all of the biopsy reports.
Key findings from the study include:
- Seven independent readers blinded to clinical and biopsy information conducted 12,283 mass reads.
- In the intent to diagnose (ITD) population 1808 masses were evaluated in 1,739 subjects (678 malignant, 889 benign, 190 benign with 12-month follow-up).
- In the ITD population of 1,739 subjects and 1,808 masses, diagnostic specificity for benign masses was 43% for OA/US and 28.1% for US, corresponding to a 14.9% absolute specificity advantage for OA/US over US (p<0.0001).
- Subgroup analyses showed no significant differences in specificity for either OA/US or US based on breast density, mass size, palpability, distance from nipple and depth.
- Specificity of OA/US was 8.4% higher in patients <50 years of age than in patients aged 60 to <70 years; there was no difference in sensitivity by age group.
- For masses that were benign, OA/US resulted in downgrading 34.5% of US reads (from BR4A to <BR3 or BR3 to BR2). OA/US also resulted in upgrading 6.0% of US mass reads, for a net downgrade rate of 28.5% (p<0.0001).
- For masses that were malignant, OA/US resulted in upgrading 47.0% of mass reads classified as BR3 by US and downgrading to BR2 27.3% of mass reads classified as BR3 by US.
- Positive predictive value for masses assessed as BR4A or higher was 51.5% for OA/US compared with 46.3% for US; negative predictive value for masses assessed as BR3 or lower is 94.4% for OA/US compared with 97.0% for US.
- Ten patients reported 11 adverse events potentially related to the OA/US procedure, all of which were considered mild and resolved within a few days (7 paresthesias, 1 erythema, 1 warmth, 1 tenderness and 1 case of possible dermatitis of indeterminate origin).
“These data demonstrate that OA/US improved diagnostic specificity compared with US, resulting in reclassification of both benign and malignant lesions,” said Erin Neuschler, M.D., Assistant Professor of Radiology at the Northwestern University Feinberg School of Medicine and the co-principal investigator of the PIONEER study. “The ability to downgrade the BI-RADS assessment of some benign masses may lead to fewer false positive examinations, short-term interval follow-up studies, and benign biopsies, potentially improving the accuracy of the diagnostic work-up and reducing some of the limitations and perceived harms of breast imaging.”
“This pivotal trial supports the Imagio system as an important advancement in evaluating breast masses and more accurately differentiating malignant and benign lesions,” said Tom Umbel, CEO of Seno Medical Instruments. “OA/US offers patients and clinicians an alternative diagnostic method that may increase specificity without significantly impacting sensitivity. False positive examinations and negative biopsies increase women’s anxiety, pain and radiation exposure, and are a contributing factor in non-compliance with current breast cancer screening recommendations. The Imagio OA/US system shows the potential to positively affect these concerns and reduce healthcare costs in the process.”
The Imagio™ breast imaging system was designed and is being studied to identify two functional hallmarks of cancer: the presence of abnormal blood vessels (tumor angiogenesis) and the relative reduction in oxygen content of blood that occurs in cancer compared to benign masses and normal tissues. The technology is CE marked in Europe and the subject of a U.S. PMA filing with the FDA.
About Seno Medical Instruments, Inc.
Seno Medical Instruments, Inc. is a San Antonio, Texas-based medical imaging company committed to the development and commercialization of a new modality in cancer diagnosis: opto-acoustic imaging. Seno Medical’s Imagio™ breast imaging system fuses opto-acoustic technology with ultrasound (OA/US) to generate fused real-time functional and anatomical images of the breast. The OA/US images provide a unique blood map around breast masses while the ultrasound provides a traditional anatomic image. Through the appearance or absence of two hallmark indicators of cancer – angiogenesis and deoxygenation – Seno Medical believes that the OA/US breast imaging system will be a more effective tool to help radiologists confirm or rule out malignancy than current diagnostic imaging modalities – without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. To learn more about Seno Medical’s OA/US imaging technology and applications, visit www.SenoMedical.com.
About Breast Cancer
According to the American Cancer Society, an estimated 252,710 new cases of invasive breast cancer, along with 63,410 new cases of non-invasive (in situ) breast cancer, will be diagnosed in U.S. women in 2017. An estimated 40,610 women in the U.S. are expected to die in 2017 from breast cancer. Only lung cancer accounts for more cancer deaths in women.[1]
[1]Breast Cancer Facts & Figures, American Cancer Society (2017). Accessed on October 24, 2017: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html

Available Now
Imagio® is FDA approved for commercial distribution in the U.S. and ready for your patients.
Contact sales


